Factory wholesale Face Mask For Health - Medical Face Mask, Type I – Shanyou
Factory wholesale Face Mask For Health - Medical Face Mask, Type I – Shanyou
Factory wholesale Face Mask For Health - Medical Face Mask, Type I – Shanyou Detail:
Product Parameters
|
REF/MODEL |
Mask Size |
Standard |
Sterilization |
Package |
|
FMA FMC |
175X95mm |
EN 14683 Type I |
Non-sterile |
25pcs/bag, 50pcs/box, 50boxes/CTN (2500pcs); 51x41x47cm (FMA) 51x41x55cm (FMC) |
|
I-S-A I-S-C |
175X95mm |
EN 14683 Type I |
Sterile |
10pcs/bag, 50pcs/box, 50boxes/CTN (2500pcs); 56*41*54.5cm (I-S-A) 56x41x59.5cm (I-S-C) |
Intended use
Medical Face Masks Type I are only used for patients and other persons to reduce the risk of spread of infections particularly in epidemic or pandemic situations. They are not intended for use by healthcare professionals in an operating room or in other medical settings with similar requirements.
Warning
● Do not use if package has been previously opened or damaged.
● Avoid touching the inside of the mask with your hands.
● Single Use Only. The recommended use time is 4 hours.
● If the Face Mask is wet or contaminated by the patient’s blood or body fluid, replace it in time.
● Dispose of used product in accordance with applicable regulations.
● Store in well-ventilated, dry place. Prevent heavy pressure, direct sunlight, hard objects
Enterprise Strength
Hangzhou Shanyou Medical “WORK” owns hundreds of mask production lines, and nine state-of-the-art extremely high speed automatic production lines. The capacity for “WORK” face masks is 10 million pcs/day, that’s 300 million per month. We’ve been exporting to UK, France, Spain, Germany, mostly government tenders in huge quantities. Our medical face masks obtained the TUV CE certificate (CE2163), registration certificate in China, SGS test report and other test reports according to EN14683. The medical face masks are in the government’s white list.
Hangzhou Shanyou Medical “WORK” strictly follows international rules to manufacture our products, make sure each item meet specific regulations and customer requirements. Our brand “WORK” is widely praised with high quality items.
Package information
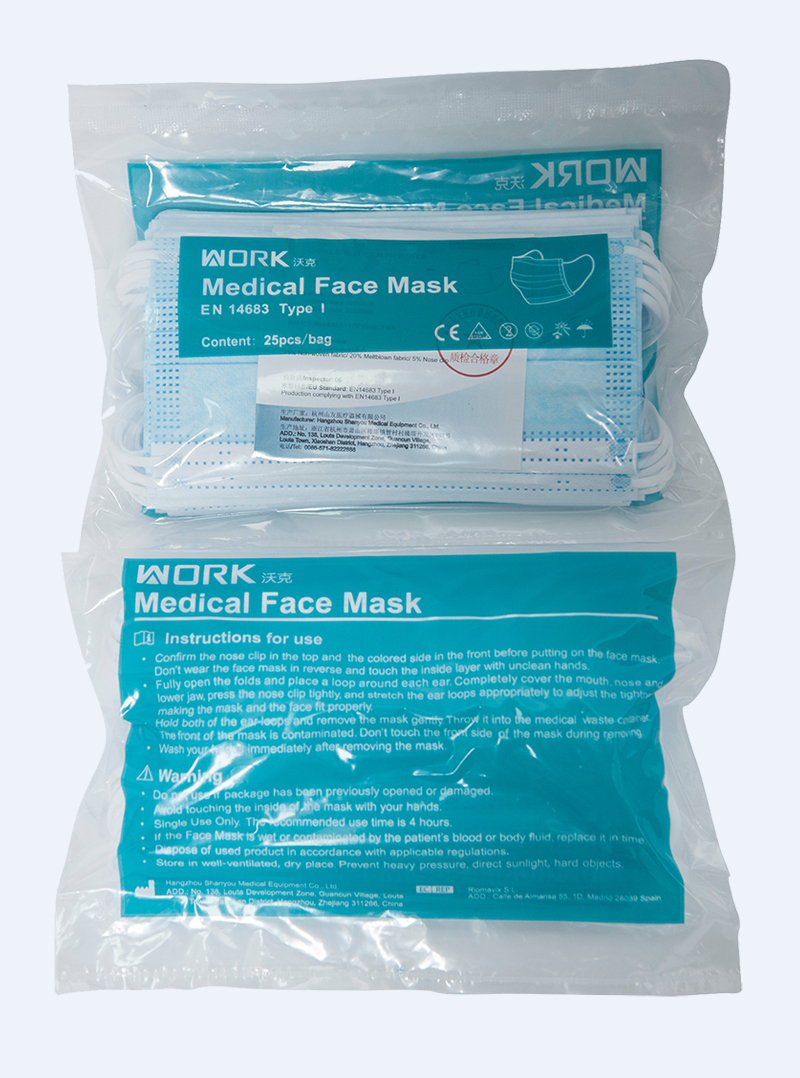


Product detail pictures:



Related Product Guide:
We regularly perform our spirit of ''Innovation bringing progress, Highly-quality making certain subsistence, Administration marketing benefit, Credit score attracting customers for Factory wholesale Face Mask For Health - Medical Face Mask, Type I – Shanyou , The product will supply to all over the world, such as: Canada, New Delhi, Guinea, We adhere to client 1st, top quality 1st, continuous improvement, mutual advantage and win-win principles. When cooperation together with the customer, we provide shoppers with the highest high-quality of service. Established good business relations using the Zimbabwe buyer inside the business, we've got established own brand and reputation. At the identical time, wholeheartedly welcome new and old prospects to our company to go to and negotiate small business.
Perfect services, quality products and competitive prices, we have work many times, every time is delighted, wish continue to maintain!